Matt Richtel who is the author of this book decided to write when he saw a miracle happen.
Jason Greenstein his close friend, who’d been on the edge of surrendering to cancer, was cured. Jason was able to cure the uncurable all thanks to a new drug that fortifies the powers of the immune system.
That was when the author decided to write An Elegant Defense. The apparently miraculous cure of his friend had kindled his imagination. If we could interfere with the human immune system and tap into its remarkable power – and with such astonishing outcomes! – then what type of illness couldn’t we defeat?
In this book, Richtel explained the history of immunology –which is the aspect of medicine that deals with the immune system. Also, he told the history in an unusual manner, combining it with the personal stories of four different individuals whose health conditions fit together with developments in the field.
In these chapters, we’ll follow Richtel’s path and take a swift journey of that history. Afterward, we’ll concentrate on one of those stories which particularly the story of Jason’s and how it has an essential lesson to teach us about both the progress and the restrictions of our ability to lead and command our elegant defense.

1 – The life within you is like a huge festival – and your immune system is in control of emitting dangerous gate-crashers.
Thinks of a massive party – a huge, rip-roaring carnival with a lot of attendees. Where is this wild, huge bash with a guest list well over a hundred times greater than the human population on Earth?
It’s within you. These merrymakers are your cells, as well as a lot of bacteria and viruses. This party is what the author names the Festival of Life.
At this festival, the gatekeepers and handymen, security personnel and emergency responders are ensuring that everything goes well– these are the cells that make up your immune system. These are your body’s elegant defense. They solve tissue damage and clean up toxins. Also, they fight off pathogen which is malicious invaders.
Pathogens are agents that cause disease and they exist in three forms which are bacteria, viruses, and parasites. We’ll be concentrating on just the first two for the purposes of these chapters.
We can consider them as tiny little killers in their greatest dangerous states. They’re very small. A few thousands of bacteria can fit inside a single. Viruses are even smaller. A few thousand would fit in one bacterium.

However, here is a quick caution: although some bacteria and viruses can be pathogenic, the majority aren’t. As a matter of fact, only one percent of the entire bacteria are likely to cause disease. Some viruses are essential for our survival. For example, approximately eight percent of our genetic material was formed from retroviruses which is a special range of viruses that attacks human cells and basically become part of our DNA.
Therefore, viruses and bacteria aren’t very bad – far from it. However, a bit of them is actually deadly.
Let’s consider the bacterium known as Yersinia pestis. It’s the cause of the Black Plague, which caused the death of 30 percent and above of Europe’s population during the fourteenth century. Some other horrible bacteria such as salmonella, E. coli, and tetanus bacillus, while the long list of deadly viruses comprises Ebola, HIV, smallpox, flu, and rabies.
Before 1900, influenza (which is a viral infection) and pneumonia (an inflammation that can either be viral or bacterial) were the main killers, causing more deaths out of every 100,000 patients than any other disease. Currently, it’s unusual to hear of someone dying as a result of the flu.
How did we conquer these pathogens? You’re going to get a lot of answers to that, which is the story of the detection of the immune system.
2 – Three mystifying findings laid the basis for the field of immunology.
The field of immunology can be found back to a mystery – or, slightly, three mysteries which are the mysteries of a chicken, a dog, and a starfish. Let’s begin with the chicken.
During the sixteenth century, one day while cutting a chicken, Fabricius ab Aquapendente a young Italian anatomist found a mysterious organ. Found below the bird’s tail, and formed like a pouch, it had no function.
He called it the bursa, a word that’s etymologically correlated to the English word “purse.” However, what was the function of this tiny avian purse? No one knew.
Nearly a century after which was in 1622, another Italian, Gaspare Aselli, cut up another animal which was a dog this time around – and he found another mystery. He saw a “milky veins” in the animal’s stomach a finding that didn’t taunt with the seventeenth-century comprehension of the circulatory system. Wasn’t blood meant to be red in color? Still, no one could describe this oddity.
Over two centuries after, in 1882, still another finding was made on Italian soil – although the originator this time was a Russian zoologist named Élie Metchnikoff. While he was visiting his sister in Sicily, he had his eureka moment.
One day, while he was working on his own, Metchnikoff put a splash of starfish larvae under his microscope. Inside these tiny, transparent organisms, he noticed what he named “wandering cells” –that appeared to float around the bodies of the starfish.

His brain made a sudden, vivid jump. What if these wandering cells weren’t as useless as they seemed? What if they were wandering watchmen, accountable for protecting the starfish against invaders?
He tested his assumption instantly. He took some rose thorns from the garden and put them beneath the skin of a few starfish larvae. Then he went to sleep.
The next day in the morning, he noticed that the wandering cells had assembled around the thorn – and that they were actively eating the damaged tissue. Metchnikoff’s experiment produced the phagocyte theory – “phagocyte” is the Greek word for “devourer of cells” – which postulates that, when attacked, the body’s initial response is to crowd the breach with these devouring cells. This cellular self-consumption is what the public name as inflammation.
However, how did the cells realize that there had been an attack?
Try to answer this question – and to describe the mysteries of the white-blooded dog and the bursa of Fabricius –led to the field of immunology.
3 – Dr. Jacques Miller found that the thymus is very essential.
During the late 1950s, Dr. Jacques Miller had many dying mice. Anytime a new mouse died – and that occurred often – Dr. Miller would dissect it and see the exact thing happened: the liver was enclosed in lesions. Every mouse had been shrunken by extreme infection.
What occurred to these poor mice? So who is this Dr. Miller?
He was a French physician that was working in London, and these mice lacked an organ that, until his experiments, was as strange as the bursa of Fabricius. This organ is known as the thymus.
After the atomic bombings of Nagasaki and Hiroshima happened, researchers worldwide started to study leukemia, occurrences of which had increased rapidly in those cities. Crucial to that research were – shock, shock – mice.
In an unpleasant nutshell, researchers exposed the mice to cancer through radiation, expecting to hit upon findings that might aid irradiated Japanese citizens. An oddity occurred during this research: some mice that hadn’t been irradiated got leukemia suddenly. This sudden advancement happened in the thymus, a tiny organ found above the breastbone.
Dr. Miller, who’d decided to examine leukemia during the 1950s, was, by the late 1950s, on the edge of making a finding that would change this unassuming organ into an essential player in the immunological field.
Dr. Miller injected mice with leukemic filtrate during an early experiment, the leukemic filtrate is a liquid-filled with ground-down cancerous tissue from a leukemic mouse. He noticed something mysterious after injecting both the baby and adult mice with this filtrate: the baby mice would get leukemia while the adult mice wouldn’t. Why is this so?

In order to know, he started removing the mouse thymuses. In the following experiments, he injected a newborn mouse with leukemic filtrate and stayed till the mouse to become an adult, and then changed its mature thymus with the thymus of a baby mouse. He performed this with a lot of mice, and, without fail, they had leukemia immediately they were given the immature thymus.
He anticipated he was on to something. The thymus-less baby mice made him sure.
As you’ll recall, these poor mice died as a result of infection, against which they’d obviously been rendered completely defenseless. Dr. Miller postulated that the thymus wasn’t a useless strange organ; it was a main part of the immune system.
4 – Dr. Miller assumed that T. cells are gotten from the thymus.
What is self and what is unknown to self? What is part of you and what is other? For example, how does the body identify which bacteria to fight and which bacteria to leave?
During the research of Dr. Jacques Miller, there weren’t any final answers to those questions, however, there was one thing that was sure which is the immune system could differentiate between self and non-self, although occasionally it would confuse help for harm.
For example, it was understood that skin grafts frequently went extremely wrong. Although at first, it might appear to embrace the new skin, the human body would eventually not accept the foreign tissue.
This understanding assisted Dr. Miller to confirm his thesis that the thymus was important to the immune system.
The body of a typical mouse, with a thymus, would, just like a human being, not accept skin transplants from other mice. There was no rejection when Dr. Miller implanted skin to the mice of the thymuses he’d removed. Certainly, he was successfully able to transplant skin from four different mice onto a single mouse, which resulted in growing tufts of multicolored fur.
Having a thymus equated skin rejection. The absence of thymus signified no skin rejection.

After his observation, Dr. Miller did a series of blood tests on these thymus-less mice, and he discovered that the entire mice had an extremely low number of a specific type of blood cell which is called the lymphocyte and it only contains one nucleus. He assumed that the thymus was the one in charge of making these lymphocytes, and then he named them thymus-derived cells or T cells in short form.
However, the question was still how these T cells could recognize and fight pathogens.
Back then during the year 1891, Paul Ehrlich an immunology inventor had presented one theory. He believed that specific cells of the human body had a type of biological set of “keys” – he tagged side-chains – that could fit into matching “locks” in the pathogens. Once the human cell got the accurate key and fitted it into the pathogen’s lock, the body could start attacking the invader. He called these the keys antibodies and he called the locks antigens.
Dr. Miller believed that he’d found the cell essential to the immune system – the correspondent of the special key-carrying cells proposed by Ehrlich during the nineteenth century. This hypothesis –similar to Ehrlich’s theory –played out to be partly accurate. The entire truth was even more amazing and difficult.
5 – Unclear evidence made researchers assume that there are more lymphocytes other than the T cells.
In 1954, the bursa which is the pouch-shaped organ that had extremely confused the young Italian Fabricius ab Aquapendente eventually acquired its moment in the scientific limelight. A researcher found that the removal of bursa from chickens greatly affected the animals’ ability to release antibodies.
This means that the removal of the bursa essentially lessened the number of antibodies. Why was this a huge issue?
It’d been found earlier that decade that the absence of antibodies signified a big problem.
In the year 1951, a severely sick boy was taken to Bethesda at the Walter Reed General Hospital in Maryland. After performing different tests, the doctors detected that the boy wasn’t producing antibodies – and they were aware that he was very sick. He’d experienced pneumonia 18 times in a lot of months, as well as a number of other lethal infections.
This clearly showed the doctor that if you lack antibodies, you were in for a terrible time. Back then, during the early 1950s, the specifics of how antibodies function wasn’t known then–however, it’d been proven that they’re present on lymphocytes which are those blood cells that have just a single nucleus.
The boy’s thymus was good, his blood had lymphocytes and his body could protect itself against some viruses. Meaning, all the identified parts of the immune system were working.
Hence, what was wrong with him precisely?
The mystery of this boy’s incident went on into the next decade.
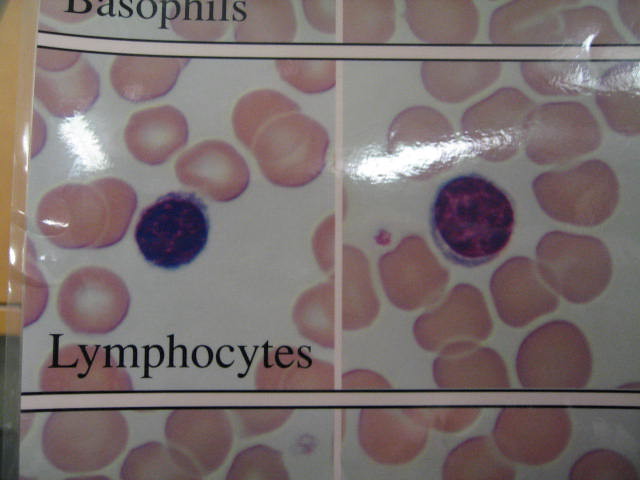
During the mid-1960s, a physician called Max Cooper started learning a rare disorder known as the Wiskott-Aldrich syndrome. People with this disorder were similar to the boy without antibodies, very prone to infection. Just like the boy, their immune systems hardly worked.
Dr. Cooper started performing autopsies on people who’d had Wiskott-Aldrich syndrome, and, strangely, they had enough lymphocytes and activity working thymuses.
Then Dr. Cooper had a sudden revelation that there are two kinds of lymphocyte,– and each one has its own root. As at then, Dr. Miller had displayed that T cells originate from the thymus. However, there had to be one other type, and it had to stem from another place.
Say you’re waving your hand in the air, saying, “I know, I know – the bursa of Fabricius!” then be prepared for disappointment. Because there’s no bursa in humans.
6 – The body’s actual defenders are the T cells and B cells.
After another various text, Dr. Jacques Miller verified Dr. Max Cooper’s hypothesis in the end
He found out that there are actually two kinds of lymphocytes known as the T cells and B cells – and the B cells which is found in the bone marrow. These cells form nearly 40% of human white blood cells –these cells, in addition to interpreting the mystery of Gaspare Aselli’s white-blooded dog, are totally essential to survival.
In order to describe how B cells and T cells function, let’s assume that you’ve been infected with the flu.
During infection, your body reacts without accuracy. A group of cells – the cells that form the other 60% of your white blood cells –quickly goes to the place of infection. It’s exactly those cells that Élie Metchnikoff noticed clustering inside the wounded starfish larvae.
At this moment, your body is not actually aware of what it’s defending itself against. This is where the T cells and B cells come in play: they give accuracy.
T cells are enclosed in tiny spikes, and few of these spikes can identify pathogens. When such occurs, a T cell can either fight the pathogen itself or send B cells to fight.
B cells have antibodies. On the surface of the B cell is an antibody that has a protein molecule and can best be illustrated as either an antenna or a key.
Recall Paul Ehrlich’s side-chain theory – the notion that specific cells have a group of “keys” that can fit into pathogens’ “locks”? The reality is a lot of foreigners. Each B cell and your body has a lot of them – is fitted with just a “key” or “antenna” (which is the antibody) that fits the precise “lock” or “signal” (the antigen) of a single organism.
How does a certain antibody discover its certain antigen? They basically have to run into each other. A B cell might wander in your body for several years – or for life – without discovering the antigen to its antibody.
How would this appear in the situation of you and the flu? The virus displays a specific antigen, and the moment a B cell with the correct antibody sees it – they join together, and the B cell damages it or produces a wave of other defenses. If you’re thinking about how your body recognizes which antibodies to combine with, then stay still, because you’re about to discover.
7 – Susumo Tonegawa learned how our bodies can identify an infinite range of antigens.
Picture yourself in a foreign country. During your stay there, you swim in a lake. A little water enters your nose, and in that water, there is a malicious bacterium that either you or any of your ancestors have ever been exposed to. The antigen it shows is totally foreign to your whole system.
How can your body probably have an antibody that matches it?
Now, meet the Japanese researcher called Susumu Tonegawa, who, in the 1970s, while working at the Basel Institute for Immunology, found what the author names the infinity machine.
At this point, researchers had recognized something apparently obvious. Let’s assume that you took a specific organism –for instance, a T cell – and isolated a specific part of its genetic material, this part would fit perfectly with the exact part of the genetic material of the other T cells.
Tonegawa had been using the B-cells to do that– mostly with B cells that were not matured. Anytime he compared a certain section of an immature B cell to the exact section in another immature B cells, he got an expected outcome; that is the sections fitted perfectly.
Then the discovery occurred. Tonegawa did the exact thing, instead of comparing the developing B cells with each other, he compared immature B cells to mature B cells. What he noticed was extraordinary.
There was a transformation in the genetic material. No other cells work like this–only B cells. The genes that code antibodies are exceptional, in the sternest sense of the word.
How does this make your body identify a foreign bacterium? This is how it does:
Every immature B cell has the same few vital genetic materials– however, in addition to that material, there’s a lot of other genetic material. As the B cell develops, a lot of that other genetic material declines. Here’s the plot: each and every cell loses a different type of genetic material. A massive pack of genetic coding homes in on a particular genetic strand.
This process makes our bodies to generate trillions of diverse antibodies. Let’s utilize the lock-and-key representation again; our B cells have keys so rare and exact that they may never find their lock. Truly, there might actually be no such locks.
By creating an infinity of solutions, our bodies can tackle the infinite threats of the world – and also some.
8 – There are two types of immune systems; when joined together, they control how our body protects itself.
A range of defenses occurs when the body is attacked by a pathogen. You’re now in a place to know how these defenses join together.
The first is a generic defense. Do you remember the phagocytic cells that Élie Metchnikoff noticed? They appear in various forms. One of them is the neutrophil. These cells make up nearly 50 or 60 percent of your white blood cells, and they’re naturally attracted to infection.
When they get to the site of the infection, the pathogen is injected with an enzyme that damages it. The neutrophil, as if it is exhausted by this fight, starts to liquefy. A fascinating fact is that pus is made of dead, dissolved neutrophils.
Afterward, the other cells enter and start to clean up the mess that the neutrophils caused.
On the other hand, the targeted defense is taking place already: dendritic cells, which are enclosed in small branch-like protrusions (dendron is the Greek word for “tree”), take a copy of the invader to T cells, which then determines if it’s benign or malignant.
Either that occurs, or a wandering T cell or B cell meets the intruder straight. These cells are then able to fit lock into key and, if the intruder is considered dangerous, it begins a very precise defense, releasing defenders that can fight the intruder well.
However, this brings about another mystifying question which is how can T cells and B cells identify harm from good? You are aware that the B cells have antibodies that are able to bind well with any antigen. However, it’s not just pathogens that show antigens; it is also shown by friendly bacteria as well.
Therefore, how does your body distinguish between the two?
There was an answer to this question in the 1990s, after two inventive researchers named Ruslan Medzhitov and Charles Janeway, found that we basically have two immune systems.
You’re now aware of the adaptive immune system which can learn. After it’s exposed to a specific pathogen, it recalls and turns out to be better at fighting this pathogen in the future. Although B cells and T cells can identify numerous antigens, they have no method of determining if to attack without input from a second system, called the innate immune system.
The toll-like receptor is important to this system. This receptor can be seen among other cells, dendritic cells, and its capable of determining generic pathogenic characters such as nucleic acids, which are connected to viral infections, and a kind of large molecule trait of a specific bacteria.
After a cell with a Toll-like receptor identifies a pathogenic characteristic, it sends an affirmative message to the T cells, there is an Enemy! –just like generals, start directing other cells to go and fight.
9 – Cytokines are the form of communication between cells which encourages as well as discourages the immune-system attacks.
Already, you’ve gotten a firm understanding of how the immune system functions. There’s still another component left which is how cells communicate with each other.
Your body is basically armed with an all-natural telecommunications system. It functions like this.
The cells of the body are capable of communicating with each other by releasing a protein known as a cytokine. For example, let’s say your body has been attacked by a pathogen, cells close to the attack will produce cytokines that pass information of the intruder to the other cells. This information can spread all through your whole body within mere hours.
Cytokines can be considered as messengers. However, they don’t deliver messages because they are the message. If there’s an attack from a pathogen, the message is to attack. For instance, the cytokine referred to as interferon.
Let’s assume that you’ve breathed in a virus. When a healthy cell identifies that there’s an attacker, it will produce interferon, which then makes it difficult for the virus to multiply. Other healthy cells carry on the interferon and start producing more interferon, thereby making life hard for the attacking virus.

However, here’s the thing: interferon has some hostile side effects. You know how it feels to have a virus, right? You begin to feel unpleasant. Your skin starts to aches. All of a sudden you feel drained of energy. It’s actually not the virus that makes you experience this– it’s the interferon.
By making you feel sore, hot and tired; your body is inspiring you to rest so it can start a defense.
But, not every cytokine tells your body to attack. A lot of them are present to make sure that our immune systems don’t overreact.
Those types of regulatory cytokines are known as interleukins. These cells essentially dampen your immune system from attacking.
The immune system needs to keep a fine balance. Nevertheless, it can’t get rid of malicious invaders by destroying the whole Festival of Life. It can’t support an indiscriminate attack. You will die if that happened. It has to be very cautious, targeting just the malicious organisms while protecting the remaining.
As you’ll learn in the following chapter, however, malignant organisms can use this point to their own benefit, choosing the body’s defenses and using them for their own wicked gains.
10 – Jason Greenstein battled with Hodgkin’s lymphoma which is a kind of cancer that attacks the immune system.
It’s high time we get to know Jason Greenstein. The author and Jason had been friends since childhood – and Jason had constantly been an energetic person, living life his own manner.
He was a talented athlete during his teenager, ruling the basketball court, however, he never for once boasted about his skills or ridiculed players that were less skilled than him. For instance, he encouraged the author all the time, who wasn’t as physically skillful as Jason.
He grew up to become a serial entrepreneur, driving all around the United States in his busted-up van, chasing one odd idea after the other.
Meaning Jason was alive. Hence, it was a surprise when death came around.
During Jason’s forties, he was diagnosed with Hodgkin’s lymphoma which is a cancer of the immune system.
It’s known as lymphoma because it invades the lymphatic system. It is possible that you know the lymph nodes – those little lumps that, if you press the accurate place, you can feel on both sides of your neck, underneath your jaw and in your armpits as well as other places. These nodes are like command centers for the immune cells. The lymphatic system is similar to the sequence of highways in which they can travel.
Hodgkin’s lymphoma lives in this system, deceiving your defensive cells into assuming that it’s part of them. It does this by invading B cells, changing them into malignant and then utilizing them to regulate the immune system. This is how:
The entire T cells have a receptor known as PD, which means programmed death. When it wants to, this receptor commands the T cell to self-destruct. Why would it do self-destruct? As you are aware, the immune system has to keep a balance between aggression and regulation, therefore it has to be able to damage as well as generate cells.
Hodgkin’s lymphoma cells have a ligand which is a molecule that sticks to cell receptors –known as PDL-1. Similar to cloaked killers, the malignant B cells wander around the lymphatic system, sticking to healthy T cells and commanding them to commit cellular suicide. Meanwhile, the immune system doesn’t identify these killers as foreign and safeguards them. Cell by cell, cancer claim the immune system.
This is the good news: this kind of cancer has the tendency to react well to chemotherapy. Approximately 90% of all incidences can be cured with chemo.
This is bad news: Jason is part of the other ten percent.
11 – Jason defeated his cancer, however, surrendered to the attacks of his immune system.
Jason had constantly been a fighter. Therefore, he fought. He went through chemotherapy –afterward, he went through remission and another session of chemo. When it wasn’t successful, he did a bone marrow transplant, which cleared out the cancerous B cells (as well as the healthy ones). Yet, cancer appeared again.
These techniques are upsetting in the extreme. Jason’s ability to generate normal blood cells had been seriously affected which made him neutropenic, meaning he had very low levels of neutrophils, the white blood cells that serve as the body’s initial responders. Jason was isolated, his body completely ruined. Giving up started to seem better than to keep fighting. Certainly, he was extremely weak to go through any more approved treatments.
Afterward was a miracle. Jason’s physician got one-time consent to treat him with a new drug that hadn’t been accepted for Hodgkin’s lymphoma. The drug name was nivolumab and the drug basically stops cancer’s instructions. Instead of being told to self-destroy, the immune system is told to fight.
It was far from sure that the drug was going to work. The physician told Jason that his likelihood of surviving was one in twelve million.
However, he actually survived. Prior to the treatment, Jason had had a big tumor on his back. A month after he began nivolumab, his tumor disappeared, and he’d lost 15 pounds, which is the weight of the tumor. Six weeks after he began taking the drugs, Jason was in full remission.
This should be where the story Jason finished– and, certainly, it’s the point when the author decided to write a book on the study of the immune system.
However, meddling on behalf of the immune system is as dangerous as leaving malignant cancer uncured.
Jason went through a dangerous treatment after his remission. His stem cells were changed with that of his sister’s. Basically, he got a new immune system. He appeared and felt better for a bit. Afterward, difficulties occurred.
At first, they were little difficulties, then bigger ones happened. In June 2016, a while after a year of his nivolumab treatment, he had liver failure, an indication that his immune system was fighting against his body. He suffered from severe inflammation, revealing a cytokine storm – a massive, chaotic reaction to the apparent invasion, which made the immune system overwork. It can kill you easily.
Jason died on the 10th of August 2016, overwhelmed by his own body defenses.
Although Jason’s story may not contain a moral. However, it tells us that: our immune system, which is the most powerful, elegant defense system known to humanity, can also be completely deadly. We meddle with it at our own jeopardy.
An Elegant Defense: The Extraordinary New Science of the Immune System: A Tale in Four Lives by Matt Richtel Book Review
The immune system needs to keep a gentle balance. It can kill us if it’s too hostile; if it’s not well hostile, it makes us susceptible to malignant pathogens. The field of immunology has a rich and interesting history, and its inventors laid the basis for the remarkable immunotherapies that are being created presently. Although our understanding of the immune system has improved greatly over the last hundred years, we’re far from being capable of taking charge of our elegant defense.
Don’t stress, and get a lot of sleep!
Your body releases adrenaline when you are stressed and this hormone, among other increases effects like increases your heart rate and blood pressure. Long-time ago, when humankind’s stressed mostly about animal predators, this was a good thing because adrenaline makes you vigilant and prepared to run. Currently, our stressors like work deadlines – aren’t usually life-threatening.
However, here’s the thing: adrenaline can become addictive. The author understood this personally. He got addicted to high-stress work practice, working too hard and riding the surge of stress-induced adrenaline. The outcomes weren’t satisfying. This habit disordered his immune system’s balance, and he ended up being very depressed. He got his balance again through sleeping – when you sleep and also meditate, the body closes the system that releases adrenaline. Therefore, if you’re battling with stress, endeavor to follow his path.
