You needn’t bother with a chemistry certificate to realize that this field is packed with captivating characters, unforeseen new developments, and a lot of sensational revelations. Now and again these occasions can be amusing, similar to the innovation of explosives, and on different occasions, they can be grievous, similar to the disclosure of radium’s actual nature.
While we can’t take you through every one of the 250 occasions in The Chemistry Book, we’ll treat you to some noteworthy features and astonishing subtleties. These revelations and milestone minutes length mankind’s history’s high and depressed spots – occasions that we’ll proceed to celebrate and occasions that have filled in as deplorable admonitions for people in the future.

Chapter 1 – Human accomplishments in chemistry began in the Bronze Age.
Our planet has consistently been home to astounding chemical cycles. Take the two-story-tall gems that pack collapses Mexico – the Cueva de Los Cristales. These colossal columns are a staggering case of what happens when the normal mineral gypsum is lowered in water that is being warmed up by magma and afterward goes through hundreds of years chilling off during an ice age.
The caverns seem as though something out of an odd chemistry fiction film. In any case, they’re genuine, dazzling, overwhelming instances of concoction responses that required no human association.
It’s difficult to state what the principal human compound disclosure was. Was it the main man-made fire? Or then again the first occasion when somebody utilized a plant to help mend an injury?
While copper was at that point being utilized for some essential instruments, around 3300 BCE, we found a superior, more grounded, and more tough material in bronze.
Bronze is the thing that happens when tin is added to copper. What’s more, what made this blend potential was travel and exchange. Around 2000 BCE, tin from Cornwall, in southwestern England, started to appear in the Mediterranean.

Inevitably, a portion of the additionally brave metal specialists in Mesopotamia started to explore different avenues regarding the materials they had, including lead, nickel, silver – and copper. In the end, bronze was conceived.
After some time, the Greeks would add more prompt the blend to make bronze simpler to work with, and afterward, zinc would be added to make metal. Notwithstanding the progressions from the beginning of time, bronze has consistently been the metal of decision for chimes, and it can in any case be found in the cymbals on your standard drum pack.
Around 1300 BCE, the Bronze Age progressed into the Iron Age. Be that as it may, this wasn’t because iron was viewed as a prevalent metal. Bronze is, actually, harder and far less inclined to erosion. Truly, what iron had made it work was accessibility.
Early iron innovation included warming charcoal and iron mineral, delivering a chunk of rough purified iron in the base of the heater. Pollutions were at that point, truly, worked out. This has consistently been a work escalated cycle and one that requires a ton of constrained air to keep heaters consuming at high temperatures. To get such conditions, it’s accepted that some refining tasks were occasional, to exploit repeating rainstorm like conditions.
In any case, notwithstanding this, iron purifying capacities immediately spread, however, it’s conceivable that areas as far eliminated as India and sub-Saharan Africa built up the innovation freely from one another.
Chapter 2 – Antiquated chemists progressed cleaning and refinement methods, regularly with seeks after gold and unceasing life.
We’ll never know who the primary chemist was, however, we do have a name for the most punctual reported chemist: Tapputi. As indicated by a tablet dated at around 1200 BCE, Tapputi was the name of a Babylonian lady who made fragrance out of fixings like myrrh and amber.
She likewise refined her blends by warming them and gathering the fumes. In this way, we can consider 1200 BCE the main recorded reference to a purging cycle including refining and filtration.
Like iron refining, numerous societies realized how to make the scent. Be that as it may, with regards to different territories and innovations, insider facts abounded in the old world.
Up until 550 BCE, individuals like the Egyptians were utilizing water to gather up trash and gather pieces of gold. At that point came King Croesus of Lydia, and another method for refining gold. Utilizing a gold-silver compound called electrum, Lydians refined unadulterated gold.
The specific strategies for their refinement are as yet being sorted out by antiquarians and archeologists. Since this cycle was probably an undocumented Lydian mystery, much is as yet obscure. Yet, one thing’s without a doubt: they benefited as much as possible from what they had.

With the utilization of liquid lead and salt, they made coinage. The cycle may have weakened the gold substance, however by stepping the coins with legendary figures, saints, and creatures, they built up a worth that brought King Croesus a clean and consistent benefit.
We should quickly advance to the beginning of the Han Dynasty, around 210 BCE. Here we find early utilization of mercury, a bizarre fluid metal that didn’t require any refining. The first to utilize a lot of mercury in a noteworthy manner was Qin Shi Huang, the unbelievable “first ruler of China.”
You might be comfortable with the enormous underground multitude of warriors, formed out of earthenware, that the sovereign had made for his burial place. Indeed, that burial place additionally comprised of a scale reproduction of his castles, total with a small waterway of streaming mercury.
Amusingly enough, Qin Shi Huang may have utilized meds containing mercury in a misinformed endeavor at eternality. We presently realize that mercury is without a doubt noxious, particularly in intensifies that permit the body to all the more promptly assimilate it. Tragically, it would be quite a while before this was known, and mercury kept on being a restorative element for quite a while.
Chapter 3 – Some antiquated procedures took many years to comprehend, while others stay a puzzle.
We should go from the beginning of the Han Dynasty as far as possible, carrying us to around 200 AD. This is the point at which we see the presence of genuine porcelain.
Before this date, there had been a wide range of noteworthy pottery, however nothing very as excellent as porcelain. Making it requires a blend of bone debris, ground glass, quartz, alabaster or feldspar, and kaolin mud, which initially originated from the town in southwest China that shares its name. Porcelain additionally requires the perfect measure of water and very significant levels of warmth.
The exact proportions and estimations were a firmly held mystery, even as the creation of porcelain increase in the hundreds of years that followed. By the 1300s, porcelain at long last advanced toward Europe, yet, nobody outside of China realized how to reproduce this perfect workmanship.
In any case, it wasn’t for the absence of endeavoring. Finally, in 1708, a detained chemist in Dresden, named Johan Frederick Bottger, along with the doctor, physicist, and logician Ehrenfried Walther von Tschirnhaus, deciphered the code.
The advancement came when the couple was at last ready to get their hands on imported kaolin dirt and alabaster. The accomplishment won Bottger his opportunity, alongside a position at the top of another porcelain industrial facility.

By around 800 AD, a ton of logical headway was occurring in Islamic and Chinese societies. One of the pioneers in this field was a man referred to in the West as “Geber.” His complete name was Abu Musa Jabir ibn Hayyan, and he lived in what is currently Iraq. “Geber” rehearsed speculative chemistry just as numerology, soothsaying, and medication.
The sacred goal for ibn Hayyan, and numerous different chemists who’d follow afterward, was the thinker’s stone. He accepted that any metal could be separated and transformed into another metal if just we had the correct solution to get this going. This remedy came to be known as the logician’s stone, and it would be some time before genuine researchers would surrender the pursuit.
In any case, a ton of what we think about Geber gets muddied by the way that his work pulled in bunches of supporters who composed original copies utilizing his name. A lot of these composing utilized images and coded language that is almost difficult to interpret. Indeed, this weird catalytic language is the place we got “gibberish.”
Chapter 4 – Great and idealistic aims can some of the time lead to unintended outcomes.
While ibn Hayyan was attempting to transform iron into gold, alchemists in China were likewise endeavoring to change metals. All things considered, these endeavors and the proceeded with journey forever expanding elixirs brought them something different completely: black powder.
The primary indication of black powder arrives in a Taoist book, dated around 850 AD. By 1044, China’s military had various plans for dangerous items.
One could nearly say it was simply an issue of time, since two of the fundamental fixings, sulfur, and charcoal, made certain to be around most chemist labs. The missing fixing is the oxidizer: potassium nitrate. This could have been included by utilizing the mineral niter, also called saltpeter, or it could have been found in caverns, around the edges of bat guano droppings. Whatever the case, when found, its dangerous potential would have been quick.
The moniker for black powder inevitably became “Chinese day off,” it would remain a military mystery for quite a while until the extensions of the Mongol realm spread this mystery to other world domains. By 1326, the main European-made firearms would perpetually change the standards of fighting.
Explosive didn’t end up being a daily existence broadening mixture, however, by the sixteenth century, a few headways toward this path were being made. In 1538, the field of toxicology got off the ground because of the endeavors of Swiss chemist and logician, Paracelsus. While most chemists were still focused on making gold and silver, Paracelsus said his goals were “to think about just what excellence and force may lie in drugs.”

As far as concerns him, Paracelsus was among the first to perceive the impacts outside specialists might be having on human wellbeing. His investigation of diggers drove him to recommend that poisonous fumes, and not malevolent mountain spirits, may be causing their lung issues.
In 1540, the German botanist and doctor Valerius Cordus blended ethyl liquor in with sulfuric corrosive to think of diethyl ether. That very year, Paracelsus distributed a composition taking note of the impact that ether exhaust had on creatures, making them oblivious, which he thought would be put to human use in time.
He was right: during the 1840s, ether turned into the principal careful sedative, just as the reason for “ether skips around” among careful understudies.
Chapter 5 – In the seventeenth century, world-changing improvements went before a move to hard chemistry.
Another significant achievement throughout the entire existence of medicinal chemistry showed up in 1631. That year, Jesuits had gotten back to Rome after an excursion to the New World, and they brought back a mind-blowing new medication. It was a compound gotten from the bark of South American cinchona trees. The medication would come to be known as quinine.
Rome was experiencing endless instances of intestinal sickness consistently, yet individuals still couldn’t seem to connect these cases to the city’s mosquito-invaded swamps. The vast majority connected the reason for “awful fumes,” which is the thing that mal-aria implies.
The Quechua individuals of Bolivia and Peru had been utilizing the tree covering to treat manifestations, for example, shuddering and chills – two of the markers of intestinal sickness.
And keeping in mind that quinine fills in as a muscle relaxant, it later ends up being phenomenal at battling intestinal sickness. It turns out the medication pursues the intestinal sickness parasites legitimately. What it does precisely remains something of a riddle, yet quinine was a distinct advantage.
Somewhere in the range of 1620 and 1630, Spain got mindful of quinine’s advantages through Jesuit evangelists. European provincial forces could now wander forward with security against the savage components of the strange world.
In any case, on the positive side, quinine turned into a much-examined compound. For a considerable length of time, those attempting to orchestrate the medication-assisted with propelling the field of natural chemistry. After German chemist Paul Rabe incompletely combined it in 1918, American physicists William von Eggers Doering and Robert Burns Woodward turned into the first to accomplish all out amalgamation in 1944.

Surely, starting in the seventeenth century, some incredible progressions were made in chemistry. What’s more, speculative chemistry at long last took a rearward sitting arrangement to a developing field of hard chemistry.
In 1661, Robert Boyle distributed The Skeptical Chymist, which successfully established the framework for current chemistry. Rather than taking a gander at things from the traditional perspective, which included the Greek idea of the four components – air, earth, fire, and water – Boyle progressed the hypothesis of molecules as the primary segment of everything being equal.
He additionally recommended that the development and responses on the nuclear level could clarify our general surroundings.
A huge number of his forecasts would wind up being right on target. Also, luckily for Boyle, the Age of Enlightenment was simply beginning, and another period of chemistry and the reason was prepared to grasp his thoughts.
Chapter 6 – In the eighteenth and nineteenth hundreds of years, the synthetic union kept on progressing.
If you’ve at any point put paint onto a canvas, there’s a decent possibility you’ve known about the shading Prussian blue. Be that as it may, you might be amazed to discover how beautiful the historical backdrop of Prussian blue is, and the critical job it played throughout the entire existence of chemistry.
Preceding 1700, blue was an uncommon shading in European artworks. This is because it was overly costly. The main dependable wellspring of blue oil paint originated from lapis lazuli stones that were sourced from Afghanistan.
At once, there was an “Egyptian blue,” yet the formula for this shading was lost someplace in the breakdown of the Roman domain. In this way, if a figure in the artwork was finished with the shading blue, you could be certain that the individual was of the most elevated status.
At that point, in 1706, German color creator Johann Jacob Diesbach made an amazing disclosure. He was attempting to make another red shade by utilizing cochineal, which originates from squashed up bugs. Be that as it may, since the reagents he was utilizing were defiled, Diesbach wound up with blue rather than red. Before long, oil paints with the name Prussian blue and Berliner blue were being sold.
However, that is not the finish of the story. While the fundamental formula for Prussian blue was spilled to the Royal Society of London in 1724, separating the chemistry behind the substance was substantially more troublesome. It was more than 250 years after the fact, during the 1970s, that the whole concoction profile of Prussian blue was completely perceived.

Like quinine, this test was a shelter for natural chemistry. En route, it yielded hydrogen cyanide – named “prussic corrosive” after Prussian blue – just as a medication to treat metal harming.
In any case, it wasn’t some time before blending wound up at the core of a disruptive issue among physicists and researchers as a rule.
In 1828, German chemist Friedrich Wohler effectively integrated urea, a generally straightforward biomolecule found in pee. What’s disputable about that? Indeed, Wohler made something that had already just been made by living animals, and he did it utilizing altogether non-natural materials like mercury cyanate.
This started a discussion around the subject of vitalism, and it’s one that may never be completely settled. The individuals who grasp vitalism accept that there’s something extraordinary and uncommon to living things – a quintessence or soul that non-living things must need. Be that as it may, here was Wohler’s urea blend, testing this general thought.
Chapter 7 – Much of the time, milestone occasions required various disclosures.
Do you recognize the name Christian Friedrich Schönbein? Except if you’re now knowledgeable throughout the entire existence of chemistry, you’re likely new to this German chemist. However, he’s assumed a part in some significant and still profoundly pertinent revelations.
One day in 1832, Schönbein was tidying up a spill at the lab and utilized his cotton cover to clean up what was nitric and sulfuric corrosive. No damage was done, isn’t that so? In any case, at that point, Schönbein put the cover by the chimney to get dry. It wasn’t some time before an unstable glimmer set his cover burning.
While he may have lost a cover, Schönbein proceeded to find nitrocellulose, which is the thing that gets made when cotton is treated with nitric corrosive. This before long turned out to be otherwise called guncotton. Individuals were promptly keen on a potential option in contrast to black powder, however, guncotton ends up being capricious and risky.
In any case, later, in 1947, the Italian physicist Ascanio Sobrero chose to perceive what happened when, as opposed to cotton, he nitrated glycerine, a sweet sugar. What he made was, obviously, nitroglycerine, and it was so hazardously touchy that Sobrero attempted to stay quiet about the outcomes for quite a while.

Notwithstanding, one physicist near Sobrero, Alfred Nobel, heard about nitroglycerine, and he got resolved to settle it. It turned out, everything that required to happen was for the nitroglycerine to be ingested into another material. The outcome was explosive.
Presently, how about we take a gander at another of Schönbein’s commitments to chemistry. In 1840, he found ozone.
While leading lab tests that included running an electrical flow through water, Schönbein saw an odd smell. He accepting the scent as indications of another substance and called it ozone, after the Greek word dozen, which means to smell.
You may have smelled what Schönbein did each one of those years back if you’ve at any point been close to a lightning storm and saw a specific “natural air” smell. Lightning, similar to the electric flow with which Schönbein was testing, additionally creates ozone.
Be that as it may, ozone is a gas, and it’s a poisonous one. Notwithstanding, its quality in the upper climate is significant and supportive. Ozone retains and shields life on the planet from the risks of bright light.
Chapter 8 – History is loaded with irksome substances.
Certain substances are continually going to be hazardous. Once in a while, there are approaches to supplant these substances with something better, however different occasions, the advantages exceed the dangers.
On account of mirrors, the hazardous material wasn’t in any event, doing that extraordinary an occupation. Early mirrors were made through a cycle that included layering glass with tin foil that had been presented to fluid mercury. So in addition to the fact that they were destructive and possibly toxic, they didn’t work superbly of giving an intelligent picture.
Luckily, in 1856, German chemist Justus von Liebig thought of them better than ever reflect. Liebig blended a silver/amine complex with a sugar arrangement and afterward applied this blend to a glass surface. The sugar atoms would then be oxidized by the silver, which brought about the sugar turning into a dissolvable corrosive that, thus, permitted the blend to be decreased to an amazingly intelligent layer of natural silver.

There is, be that as it may, one risky catch. If the silver/amine arrangement isn’t utilized immediately, it can go through further responses that will transform it into the silver nitride. This substance is shaky to such an extent that it can detonate for apparently no explanation by any means!
At that point there’s diazomethane. This is a substance intensify that has a considerable rundown of impetuses to detonate – including daylight, warmth, and sharp edges. Accordingly, it requires colossal consideration and exceptional, cleaned crystal when being utilized. Goodness, and did we notice that it’s profoundly noxious too?
The thing is, however, diazomethane can do something amazing. It’s one of chemistry’s incredible reagents. This implies by including diazomethane, chemists can get an entire scope of responses to occur effortlessly. Truly, it’s fickle and harmful, but at the same time, it’s one of the most supportive devices in a chemist’s tool kit.
Yet, in case we’re discussing harmful substances, we need to refer to one that is inseparable from poison: cyanide. In case you’re wearing a gold ring, accessory, or watch, there’s a decent possibility that cyanide was utilized to separate and filter that gold.
In 1887, a Scottish chemist and two specialists from Glasgow created the MacArthur-Forrest measure, which includes utilizing a cyanide answer to break up gold from pieces of metal. A few spots have restricted the cycle because of the innate risk of utilizing a lot of cyanide-imbued water. Be that as it may, given how modest the cycle is, and how high the interest for gold remains, it’s despite everything utilized today.
Chapter 9 – A few revelations came at a hefty expense.
Among the greatest advancements in the mid-twentieth century was our comprehension of radioactive substances.
One of the main pieces of information came in 1896 when the French physicist Antoine-Henri Becquerel found that uranium salts could make photographic plates become uncovered. Becquerel realized that uranium mixes were transmitting a type of radiation.
Becquerel’s discoveries provoked the curiosity of Marie and Pierre Curie, who ran a lab gaining practical experience in the exploration of gems and attraction. Marie started a thorough quest for different substances that produced comparative radiation.
This drove her to the component thorium and the mineral pitchblende. Through pitchblende, she segregated two new radioactive substances, polonium, named after Marie’s nation of origin of Poland, and radium.
This work required long periods of exertion and a ton of pitchblende. It wasn’t until 1902 that their work finished in a paper by Marie that wound up being granted two Nobel Prizes. However, what the Curies didn’t understand was that consistently they were being harmed by the radiation. Indeed, the couple’s lab books remain hazardously radioactive right up ’til today. They’re put away in lead-lined boxes and require defensive garments to deal with.

In any case, it would be some time before the threat was truly perceived. In 1913, a bit of the riddle was found by two British physicists, Ernest Rutherford and Frederick Soddy. They found that radium was the consequence of rotting uranium molecules. This implied one component could have numerous structures, which Soddy named isotopes, after the Greek words iso and topos, signifying “equivalent” and “spot.”
But instead than appearing to be a risk, radioactive components previously gave indications of recuperating benefits, particularly when it reached halting the spread of carcinogenic cells and skin infections. At the point when this became obvious, a few business visionaries ran with selling radioactive sorts of toothpaste and skin creams. One such item was a tonic called ‘Radithor,’ which flaunted that each jug contained a portion of radium.
Unfortunately, this case was valid. One survivor of Radithor was the Pittsburgh steel organization proprietor, Eben Byers, who professed to drink upwards of three containers of Radithor consistently and filled in as a representative for the tonic. In 1932, he lost his life to bone malignancy and must be covered in a casket fixed with lead.
Concerning the silver coating of the story, his passing prompted a government crackdown on items like Radithor, and new laws requiring testing and endorsement before such items could enter the market.
Chapter 10 – It took a long time before the impacts of leaded fuel were uncovered.
As the account of Radithor shows, now and again benefits and chemistry lead to calamity. Another unflattering section throughout the entire existence of chemistry has a place with tetraethyl lead.
Created in 1921 by General Motors head Charles Kettering and scientific expert Thomas Midgley Jr., tetraethyl lead was added to car gas to permit the fuel to consume all the more uniformly. And keeping in mind that it did precisely that, it likewise made hurtful measures of lead be delivered through fumes vapor.
Despite numerous passings happening during the assembling of ethyl gas, as it was approached the market, Midgley swore at a public interview that tetraethyl lead was sheltered. He even held some right in front of him for emotional impact. Unbeknownst to anybody at that point, Midgley had just been attempting to recuperate from lead harming.

The full image of lead tainting wouldn’t turn out to be clear until 1965 after crafted by American land physicist Clair Cameron Patterson was distributed. Patterson didn’t decide to reveal lead pollution. He was examining the rot of uranium and lead isotopes as a method of building updating strategies. In 1956, he assessed the Earth’s age at around four and a half billion years of age – a figuring that has withstood logical investigation.
In this way, Patterson had taken examples from around the globe and dissected the lead levels. He distributed his discoveries in a book called Contaminated and Natural Lead Environments of Man.
It uncovered that the acquaintance of tetraethyl gas with vehicles and planes the world over had immediately become the main supporter of lead defilement on the planet. This wasn’t simply influencing the environment. The lead was harming water and the natural pecking order also.
The sensational increment was a lot for certain researchers to accept, however, Patterson’s information looked at, and numerous nations started to prohibit lead from gas, paint, water pipes, and different items.
Yet, that is not the finish of the story for the chemist expert Thomas Midgley Jr. In 1974, just a year after the Environmental Protection Agency started eliminating ethyl gas, we got mindful of another planet-hurting development attached to Midgley and Charles Kettering: Freon. We’ll take a gander at this story in the following section.
Chapter 11 – The twentieth century included some grievous substance improvements.
Sometime in the distant past, claiming a cooler was a perilous suggestion. During the 1920s, coolers worked by extending and packing gases. For certain units, that gas was propane. For other people, it was smelling salts or sulfur dioxide. These gases included a type of danger, be they poisonous to inhale or exceptionally combustible. Furthermore, since a considerable lot of the gases were normally destructive, a spilling fridge was a very basic event.
Freon, the business trademark for dichlorodifluoromethane, was created in 1930 to take care of this issue. It was both non-combustible and non-destructive. It was before long being utilized for various items, similar to hair shower and asthma inhalers.
In any case, more than 40 years after the fact, it was found that chlorofluorocarbons (CFCs) like Freon were expanding the degrees of without chlorine radicals in the climate. For reasons unknown, CFCs are additionally responsive to UV light – and this response prompts an unsafe cycle.
UV light makes the CFCs separate and deliver chlorine free radicals, which at that point makes ozone separate, which, thusly, delivers much more free radicals. In this way, a modest quantity of CFCs prompts huge issues. Indeed, one chlorine radical can bring about countless obliterated ozone particles.
This got evident in 1974 when American physicist Frank Sherwood Rowland and Mexican chemist Mario Jose Molina delivered their investigation on CFCs and ozone. Sufficiently sure, the layer of ozone ensuring the planet was as a rule truly exhausted, and laws were before long being passed to boycott the utilization of CFCs like Freon.
By the last part of the 1900s, it was turning out to be progressively evident that specific synthetic substances accompanied shocking results if not took care of with the most extreme consideration. One occurrence during the 1980s made this agonizingly understood.
In 1984, the most noticeably terrible concoction fiasco of its time occurred in Bhopal, India. It occurred at a Union Carbide plant that was producing a compound utilized for pesticide, known as MIC, or methyl isocyanate. This concoction can cause eye disturbance if only two sections for each million are noticeable all around. If that sum surpasses twenty sections for each million, your lungs can be harmed.
On the evening of December 2, 1984, thirty metric huge amounts of MIC spilled from the plant and secured 25 square miles of the encompassing region. The reason for the break is as yet a matter of discussion, however huge numbers of Bhopal’s half-million inhabitants endured long haul instances of eye and lung injury.
Chapter 12 – Current examination methods have prompted the advancement of life-sparing medications.
There’ve been trial old Egyptians, goal-oriented chemists in China, and diligent European chemists during the Age of Enlightenment. Since the beginning, one thing has stayed valid: we have a desire to infer mending and life-broadening drugs from the wonderful assortment of atoms on the planet.
In 1988, a Nobel prize was given to three researchers who mirror our advanced endeavors in the ceaseless journey for new meds. Two of those researchers were American partners, Gertrude Belle Elion and George Herbert Hitchings. Their creative examination strategies created compelling medications to battle intestinal sickness, malignancy, bacterial diseases, and HIV/AIDS.

What Elion and Hitchings spearheaded was the creation of purine subordinates. This is a class of intensifies that help structure biomolecules, for example, DNA. Having a purine structure gives chemists an extraordinary initial phase in making new medications, and who realizes where we’d be present without their work.
The third honoree of the 1988 Nobel prize was the Scottish doctor and pharmacologist Sir James Whyte Black. He’s answerable for two of the world’s top-rated drugs, or possibly the exacerbates that are utilized in those medications: cimetidine, which treats ulcers, and propranolol, which treats coronary illness.
Another critical advance forward in drug improvement came in 2010 when the consolidated powers of the medication organization Merck and the bio-designing organization Codexis split the long-standing objective of building proteins.
Recall that unpredictable however helpful reagent diazomethane. Having the correct protein during an amalgamation cycle can have a significant effect. It can make responses run easily, neatly, and rapidly, which is the thing that each chemist wants.
Merck’s essential objective was to improve the blend of one of its diabetes prescriptions, sitagliptin. So they got Codexis, and soon they were running a progression of PC model varieties, looking for the correct protein to carry out the responsibility. In the wake of running more than 36,000 varieties, they at long last found what they were searching for: a built protein that wound up having 27 of its amino acids changed.
This was an enormous milestone for the production of engineered medications, and it could drastically change clinical chemistry as well as the field of chemistry out and out. While protein building is still moderate and exorbitant at this moment, it’s presumable simply a question of time before improved procedures and preparing power make it more viable and typical.
In the last section, we’ll investigate two or three different improvements not too far off that could have game-evolving results.
Chapter 13 – Future achievements may include developments to lessen carbon dioxide discharges.
So what does the eventual fate of chemistry hold? What milestone occasions would we be able to anticipate in the years to come?
Bunches of invested individuals are currently seeking after a spotless vitality source. A large number of our present powers add to the nursery impact, a marvel originally found in 1896 by Swedish chemist Svante August Arrhenius. The development of carbon dioxide and water fume in the environment keeps heat from getting away and subsequently unleashes ruin on our atmosphere.
Enter hydrogen. While a significant number of the present advantageous force sources make carbon dioxide discharges, copying hydrogen just deliveries water fume, which makes it an alluring and inexhaustible fuel. Individuals have been looking to hydrogen as the fuel of things to come since in any event the 1970s. There are, be that as it may, a couple of entanglements.
In this way, hydrogen isn’t a vitality source. Power can be changed over into hydrogen through a cycle including water. Be that as it may, the genuine issue to defeat is capacity. Hydrogen particles are so little they can even ingest into metal structures, making it a troublesome fuel to store. Nonetheless, the creator accepts that this obstacle could be cleared by around 2025.
Similarly is the improvement of fake photosynthesis, which could show up in around 2030.
Basic data about photosynthesis was uncovered in 1947 when researcher Samuel Goodnow Wildman found Rubisco. This plant protein later ends up being essential for the Calvin cycle, which is a key aspect of the photosynthesis cycle that includes transforming carbon dioxide into glucose.
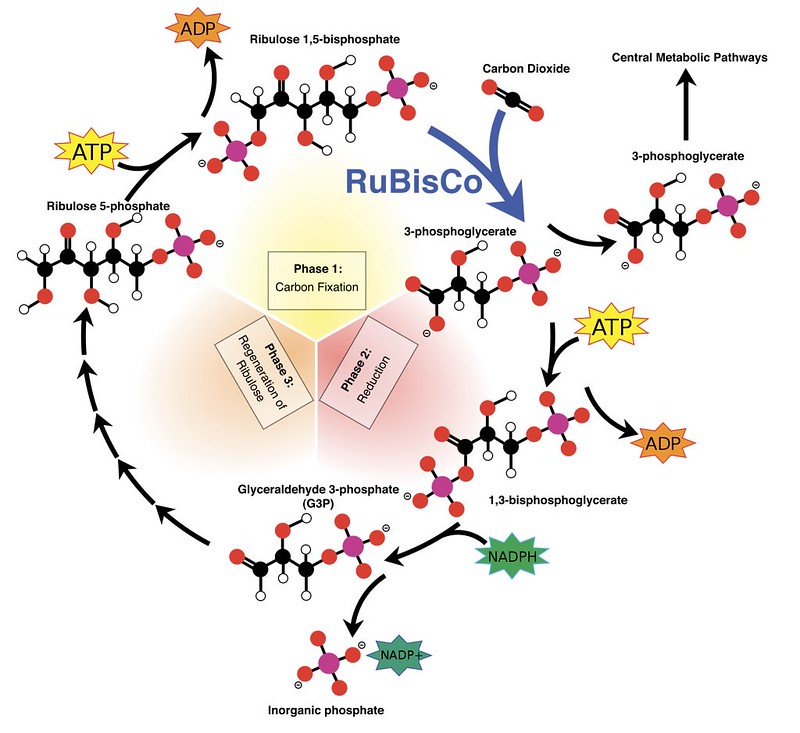
It’s an extraordinary modest representation of the truth to state that photosynthesis is critical. It delivers the oxygen that keeps us alive and directs carbon dioxide, accordingly making earth inhabitable. And afterward, there’s the way that without plants, our natural way of life would self-destruct.
Be that as it may, there’s one odd thing about the Rubisco compound: it’s truly moderate. It just experiences three sub-atomic changes every second, and nobody’s truly certain why. So the inquiry is, how might we improve the cycle? All things considered, the advantages could be world-sparing, since a quicker chemical could catch and eliminate more carbon dioxide from the climate.
There are other likely advantages to counterfeit photosynthesis, including the chance of getting hydrogen out of the water without utilizing power. The entirety of this could drastically diminish one of the fundamental drivers of the developing atmosphere emergency. The truth will surface eventually if the present chemists will have the option to enhance nature to make all the difference.
The Chemistry Book: From Gunpowder to Graphene, 250 Milestones in the History of Chemistry by Derek B. Lowe Book Review
The historical backdrop of chemistry is brimming with interesting stories and noteworthy people who’ve carried us closer to seeing all the perplexing synthetic responses that are going on around us. Huge numbers of the revelations are altogether the all the more stunning since they occurred unintentionally.
Different occasions have an unfortunate inclination since they’ve shown us the risks of certain synthetic substances. Numerous lives have been lost, however, chemistry is likewise behind a large number of the life-sparing and life-delaying headways that have been made throughout the long term.
Chemistry may at present amazement us in the years to come if researchers can figure out how to make clean powers and eliminate unsafe carbon dioxide from the climate.
